FDA recalls hundreds of products due to unsanitary conditions at Minnesota distribution center
01/23/2026 / By Laura Harris

- The FDA announced recalls of hundreds of consumer products, including popular over-the-counter medications, due to unsanitary conditions at Minneapolis-based Gold Star Distribution Inc.
- Recalls cover medications like Advil, Tylenol, Bayer aspirin, DayQuil/NyQuil and more than 800 other items, including food, pet food, cosmetics and personal care products.
- Inspections found rodent and bird droppings and urine in the facility, creating a risk of Salmonella contamination on product surfaces and packaging.
- Consumers and retailers are instructed to destroy recalled products, not return them, and provide proof to Gold Star to receive refunds.
- Salmonella exposure can cause diarrhea, fever, vomiting, dehydration and abdominal cramps; it can be severe or life-threatening for children, the elderly and immunocompromised individuals, with rare complications affecting the bloodstream, brain, joints or heart lining.
The Food and Drug Administration (FDA) has announced recalls of hundreds of consumer products, including many widely used over-the-counter medications, after inspectors found unsanitary conditions at a Minnesota distribution center.
In multiple notices posted Jan. 14, the FDA said that Minneapolis-based Gold Star Distribution Inc. initiated recalls for certain lots of popular medications, such as Advil, Bayer aspirin, Tylenol, Alka-Seltzer, Claritin, DayQuil, NyQuil, Pepto-Bismol, Pepcid Complete, Halls cough drops and Tums. Children’s cold and flu medicines are also among the affected products.
According to the agency, the recalls stem from “unsanitary conditions, including rodent exposure/activity” at Gold Star’s distribution facility. The company began the recall process on Dec. 26, 2025, while the FDA later upgraded the actions to Class II recalls, indicating that use of the products could cause temporary or medically reversible health effects, though the likelihood of serious harm is considered low.
Beyond medications, more than 800 total items distributed by Gold Star are under recall, including human food products, pet food, cosmetics and personal care items. The FDA has posted a comprehensive list of affected products on its website. Additional recalled items include several types of Colgate toothpaste, Axe body spray, Vaseline products, Lucky Ice mouthwash, Carmex products, vaporizing chest rubs, ice gels and Swan White Clear Alcohol.
An FDA news release stated that inspections uncovered bird droppings, rodent feces and rodent urine in areas where drugs, food, medical devices, pet food and cosmetic products were stored. The company cited a risk of potential Salmonella contamination due to rodent and avian exposure and unsanitary storage conditions.
The affected products were distributed to more than 50 retail stores, nearly all located in the Minneapolis–St. Paul metropolitan area. As of the initial recall announcement on Dec. 26, no illnesses had been reported.
Consumers and retailers are being instructed not to return recalled items. Instead, they should destroy the products and provide proof of destruction to Gold Star to receive a refund.
“Consumers and retailers who purchased the affected products should destroy the products as soon as possible and verify such destruction by receipt provided to Gold Star. Products should not be shipped back to Gold Star under any circumstances. Gold Star will provide refunds upon request,” the recall notice stated.
The FDA said it continues to monitor the situation as the recalls remain ongoing.
Packages contaminated with Salmonella can be transferred to hands, household surfaces or food
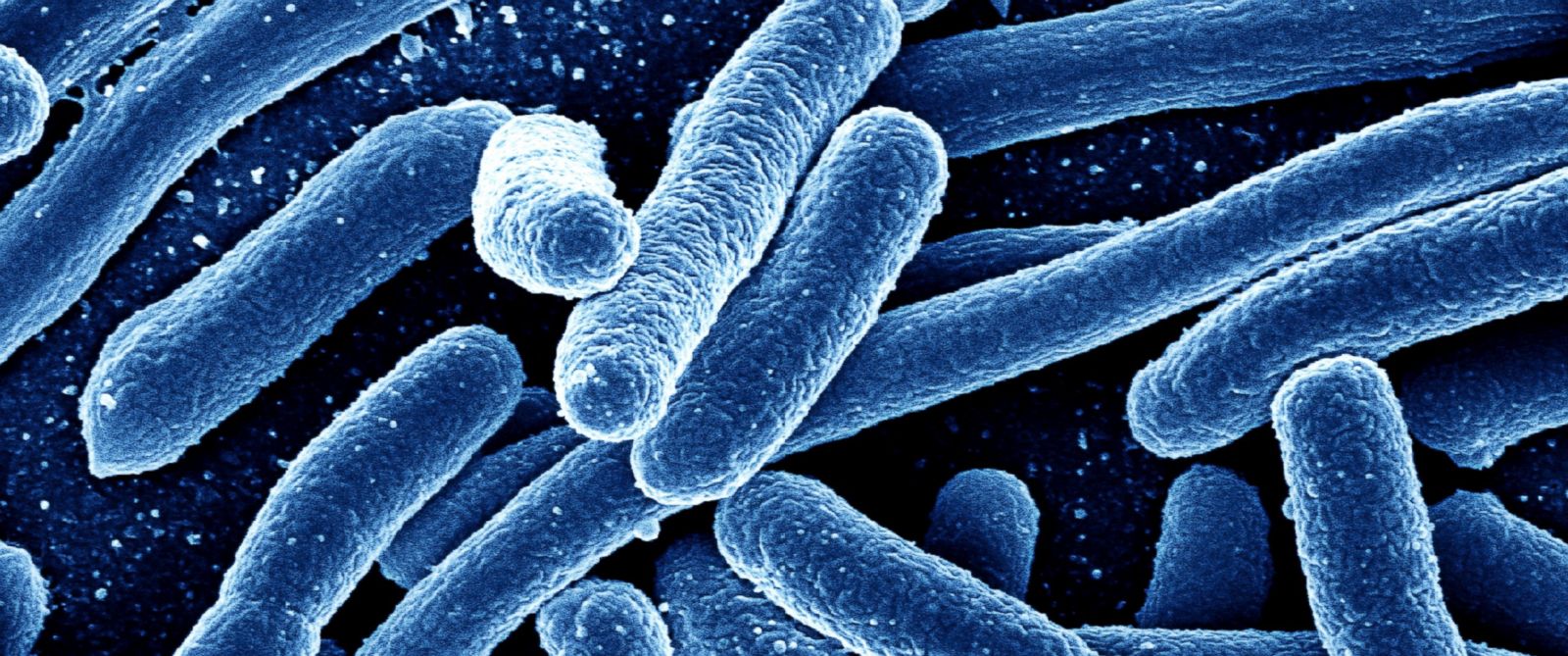
Rodents and birds, as BrightU.AI‘s Enoch noted, are known carriers of Salmonella, and their droppings or urine can contaminate storage surfaces, shelving, pallets and outer packaging of products, even if the items themselves remain sealed. When consumers or retailers handle these contaminated packages by touching, opening or storing them in homes, kitchens or medicine cabinets, the bacteria can be transferred to hands, household surfaces or food.
Symptoms of Salmonella poisoning include diarrhea, fever, vomiting, dehydration and abdominal cramps, typically beginning six hours to six days after exposure and lasting up to a week.
While most people recover without treatment, the illness can be life-threatening for young children, older adults and individuals with weakened immune systems. It can lead to serious complications, including bloodstream infections, and, in rare cases, infections affecting the brain, joints or the lining of the heart.
Watch this video of Dr. Kevin Hargin of the British Food Standards Agency explaining what salmonella is.
This video is from the Daily Videos channel on Brighteon.com.
Sources include:
Submit a correction >>
Tagged Under:
Class II, clean food watch, contamination, dangerous, FDA, food safety, food supply, grocery, Prescription drugs, Product recall, products, recall, sanitation, stop eating poison
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
PrescriptionWarning.com is a fact-based public education website published by Prescription Warning Features, LLC.
All content copyright © 2018 by Prescription Warning Features, LLC.
Contact Us with Tips or Corrections
All trademarks, registered trademarks and servicemarks mentioned on this site are the property of their respective owners.